
WHAT IS NUTRIGENOMICS?
Nutrigenomics is an interdisciplinary field of study that investigates the relationship between nutrition, genes, and health. Derived from the words “nutrition” and “genomics,” nutrigenomics seeks to understand how the foods we eat interact with our genetic makeup to influence our health positively or negatively. This is a departure from the traditional “one-size-fits-all” model of nutrition, where dietary recommendations are made for the population at large.
The importance of nutrigenomics extends beyond mere curiosity. Diseases such as diabetes, obesity, cardiovascular diseases, and even certain types of cancer have both genetic and nutritional factors that contribute to their onset and progression. By understanding how specific nutrients interact with particular genes, nutrigenomics has the potential to offer targeted prevention and treatment strategies. For instance, understanding which genes are influenced by certain dietary components can help in creating a nutrition plan that can support these genes, affecting disease risk or progression.
HOW NUTRIGENOMICS OFFERS A PERSONALISED APPROACH TO NUTRITION
The central promise of nutrigenomics is personalisation. The idea is not just to understand what foods may be generally “good“ or “bad” for health, but to determine what foods may be beneficial for your health based on your genetic makeup. With the advancement of genome sequencing technologies, it has become increasingly affordable and accessible to assess your genetic profile. This profile, combined with nutrigenomic research, can guide personalised dietary recommendations.
Imagine two individuals with a family history of breast cancer. One of them may have a genetic profile that suggests a faster rate of oestrogen detoxification, while the other may not. Nutrigenomics can potentially offer each of them a personalised nutrition plan designed to either support their natural oestrogen detox capabilities, thereby affecting their overall breast cancer risk differently.
This personalised approach extends to micronutrients, macronutrients, and various food groups, allowing for diets to be tailored to individual metabolic types, health conditions, and even lifestyle factors. As a result, nutrigenomics transforms the act of eating from a general health practice to a targeted intervention, optimising health outcomes at an individual level.
UNDERSTANDING THE LINK BETWEEN NUTRIGENOMICS AND BREAST CANCER RISK
THE GENETIC FACTORS THAT INCREASE SUSCEPTIBILITY TO BREAST CANCER
Breast cancer is a complex disease influenced by a variety of factors, including genetics. Certain genetic mutations, most notably the BRCA1 and BRCA2 genes, have been closely associated with a significantly higher risk of developing breast cancer. These mutations interfere with the cell’s ability to repair damaged DNA, potentially leading to uncontrollable cell growth and cancer. However, it’s important to note that having these mutations does not guarantee that one will develop breast cancer; it simply increases the risk.
Aside from BRCA mutations, other genetic markers, known as single nucleotide polymorphisms (SNPs), have been identified as less potent but still relevant factors that may influence breast cancer risk. These SNPs may play roles in hormone regulation, immune response, and cellular growth and repair mechanisms, among other functions.
HOW NUTRITION INTERACTS WITH GENETICS
Nutrigenomics offers intriguing insights into how nutrition can interact with these genetic factors. Certain nutrients have been shown to influence gene expression, either activating or deactivating genes that are linked to breast cancer.
For instance, nutrients like folate, found in almonds and avocados, may interact with genes involved in DNA repair. Omega-3 fatty acids from flaxseeds may play a role in downregulating genes associated with inflammation, which is a known contributor to cancer progression. On the flip side, high intake of processed foods has been linked to the activation of genes that promote inflammation and tumour growth.
Additionally, nutrients like indole-3-carbinol (I3C) found in cruciferous vegetables (e.g., broccoli, cauliflower and kale) may play a role in oestrogen metabolism, supporting the detoxification and removal of harmful oestrogen metabolites from the body.
THE ROLE OF GENES IN HORMONE PRODUCTION, REGULATION AND DETOX
Hormones like oestrogen play a critical role in various bodily functions, from reproductive health to bone density. However, an imbalance in hormone levels or their metabolism can lead to a variety of health problems, including breast cancer. Genes have a significant influence on how hormones are produced, regulated, and detoxified in the body.
HORMONE PRODUCTION
Certain genes are responsible for the biosynthesis of hormones like oestrogen. For example, the aromatase gene (CYP19A1) plays a crucial role in converting androgens into oestrogens.
HORMONE REGULATION
Genes like the oestrogen receptor genes (ER-alpha and ER-beta) regulate how the body responds to oestrogen. Mutations or variations in these genes can impact sensitivity to the hormone, potentially affecting breast cancer risk.
HORMONE DETOX
Genes related to liver enzymes play a vital role in hormone detoxification. These include the cytochrome P450 family of genes, which help convert active forms of oestrogen into less active forms that can be easily excreted from the body.
THE SIGNIFICANCE OF OESTROGEN DETOXIFICATION IN BREAST CANCER RISK
Oestrogen detoxification is the metabolic process by which the liver transforms oestrogen into less potent metabolites for excretion. This detox pathway is crucial for maintaining hormonal balance and reducing the risk of oestrogen-sensitive cancers. While the liver is the primary site for oestrogen detoxification, its metabolites are ultimately excreted through urine and faeces.
Oestrogen detoxification is particularly significant in the context of breast cancer because some breast cancers are oestrogen-receptor-positive, meaning they grow in response to oestrogen. Inadequate detoxification can lead to a buildup of harmful oestrogen metabolites that can bind to these receptors and promote tumour growth.
Additionally, specific genetic profiles may either speed up or slow down the rate of oestrogen detoxification, which can be a pivotal factor in breast cancer risk. For individuals with a slower detoxification rate, implementing nutritional strategies to support this process may be particularly beneficial. For those with a faster rate, balancing out oestrogen levels to avoid detrimental low levels may be more appropriate (1).
Nutrigenomics can provide insights into which nutritional strategies may be most effective for oestrogen detoxification, based on one’s genetic makeup. By understanding these mechanisms, individuals can take proactive steps in their diets to mitigate the risks effectively.
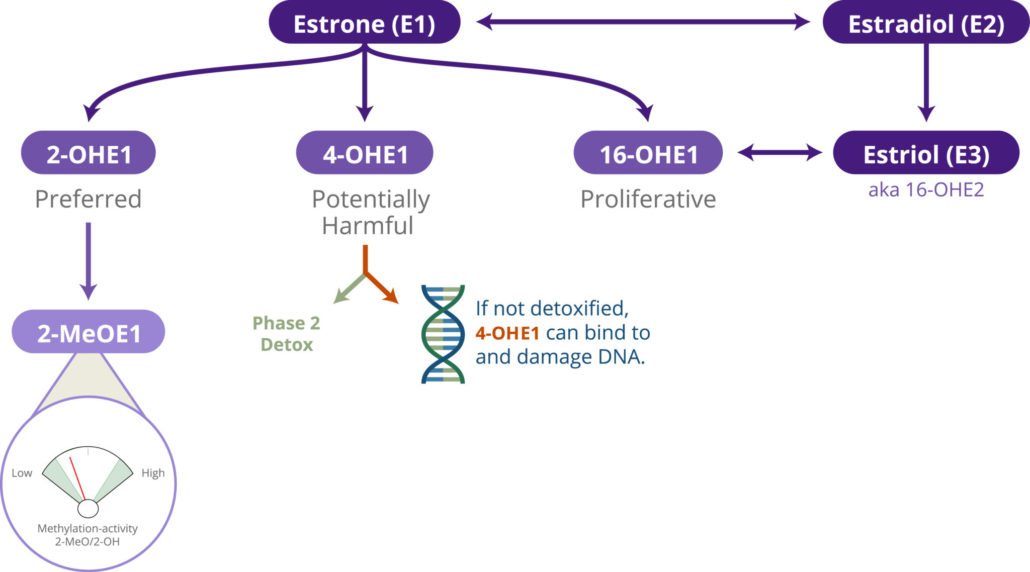
THE ROLE OF DIET IN OESTROGEN DETOX
Nutrition can be a double-edged sword when it comes to hormone detoxification. While some foods and nutrients actively support this process, others can be counterproductive.
SUPPORTIVE FOODS AND NUTRIENTS
- Cruciferous vegetables: foods like broccoli and Brussels sprouts contain compounds like I3C and sulforaphane, which aid in metabolising oestrogen into less potent forms
- Fibre: integral for hormone regulation, fibre helps in the elimination of oestrogen by binding it in the digestive tract
- Omega-3 Fatty Acids: found in fatty fish, these fats may support healthy inflammation, thus supporting hormone balance
- Folate: rich in leafy greens, folate is essential for DNA methylation, a crucial process in hormone detoxification
- Green tea: rich in antioxidants like epigallocatechin gallate (EGCG), green tea has been studied for its potential role in supporting the inhibition of breast cancer growth
- Seeds: chia and pumpkin are high in zinc and other nutrients that support hormone balance and immune function
- Nuts: almonds and walnuts contain healthy fats and antioxidants that may help support hormone regulation
- Herbs and spices: turmeric contains curcumin, which has anti-inflammatory properties, and rosemary has antioxidants that may support liver function, a key organ for hormone detoxification
- Quality proteins: lean meats like poultry, fish, and plant-based proteins like legumes can provide essential amino acids that are crucial for hormone synthesis and detoxification
- Phytoestrogens: flaxseeds and soy can offer a balanced oestrogenic activity, potentially reducing breast cancer risk when consumed as part of a well-rounded diet (2)
HINDERING FOODS AND NUTRIENTS
- High sugar and processed foods: these can induce inflammation and hormone imbalance
- Excessive alcohol: because it impairs the liver’s detoxifying capabilities
- Red meat: particularly when processed or cooked at high temperatures, red meat can disrupt hormone balance (3)
THE IMPORTANCE OF HORMONAL BALANCE
While it’s vital to detoxify excess oestrogen, let’s not forget that oestrogen itself is not the enemy. It has crucial roles in bone maintenance, skin health, and reproductive functions among others. An imbalance in either direction—excess or deficiency—can lead to health issues. High levels can risk hormone-sensitive cancers, while low levels can affect bone density and cardiovascular health.
TAILORING DIET TO GENETIC MAKE-UP: A NUTRIGENOMIC APPROACH
Understanding your genetic makeup can further refine your dietary approach. This personalised strategy enables a nuanced approach to hormone balance and breast cancer risk reduction.
ADVANTAGES OF A NUTRIGENOMIC APPROACH
- Precision: nutrigenomics allows for personalised nutrition, which is far more precise than generalised dietary guidelines
- Risk mitigation: understanding your genetic predispositions can help you make dietary choices that actively mitigate your risks for conditions like breast cancer
- Holistic well-being: a personalised diet can also contribute to overall well-being by supporting healthy inflammation, improving gut health and optimising nutrient absorption
KEY NUTRITIONAL STRATEGIES
Here are five key nutritional takeaways to consider for an effective approach to supporting hormone balance:
- Embrace cruciferous vegetables
- Prioritise dietary fibre
- Consider phyto oestrogens
- Limit inflammatory foods
- Opt for healthy fats
THE POTENTIAL FUTURE OF PERSONALISED DIET PLANS AND GENETIC TESTING
- Tailored nutrition plans: as genetic testing becomes more accessible and affordable, the prospect of creating highly personalised nutrition plans based on one’s unique genetic makeup is increasingly feasible. These plans could be as specific as outlining optimal macronutrient ratios, suitable types of exercise, and even ideal meal timing, all based on one’s genetic predispositions
- Integration with health care: nutrigenomic data could eventually be integrated into healthcare records, allowing for more holistic and targeted care. Doctors could consult this data when prescribing medications or recommending lifestyle changes, thus improving efficacy and reducing potential side-effects or contraindications
- Predictive and preventative medicine: nutrigenomics fits well within the growing trend towards predictive and preventative medicine. By understanding the genetic predispositions to various conditions, individuals can take proactive steps to mitigate risks through dietary and lifestyle adjustments
- Ethical considerations: as with any genetic information, there will be ethical considerations, such as data privacy and potential discrimination based on genetic predispositions. However, with appropriate safeguards, the benefits could outweigh the risks
- Public health implications: on a broader scale, understanding how genes interact with nutrients could inform public health guidelines, making them more nuanced and effective at a population level
Nutrigenomics has broad-reaching implications for the future of healthcare and disease prevention. Its principles can be applied to a variety of health conditions beyond breast cancer, opening the door for more personalised, effective treatments. As genetic testing becomes more mainstream, the possibility of creating highly individualised healthcare plans becomes more real, marking a significant step forward in the evolution of medicine.
WHERE DO WE GO FROM HERE? FUTURE DIRECTION AND AN ACTION PLAN
As we’ve journeyed through the complexities of nutrigenomics, oestrogen detox, and their implications for breast cancer risk and overall health, it becomes abundantly clear that this growing field offers promising avenues for personalised healthcare. Nutrigenomics is not just a scientific buzzword; it’s an evolving discipline with the potential to revolutionise the way we think about health, disease prevention, and treatment.
Knowledge is power, especially when it comes to understanding how your genes can influence your health risks and how you can modify those risks through diet. If you’re curious about your genetic predispositions, consider undergoing genetic testing from a reputable source. This can provide you with invaluable insights into how your body metabolises specific nutrients, responds to certain foods, and even how it detoxifies hormones like oestrogen. Armed with this information, you can make more informed decisions about your diet and lifestyle.
- Longcope C. Relationships of estrogen to breast cancer, of diet to breast cancer, and of diet to estradiol metabolism. J Natl Cancer Inst. 1990;82(11):896-7. doi: 10.1093/jnci/82.11.896. PubMed PMID: 2342120.
- Dikshit A, Hales K, Hales DB. Whole flaxseed diet alters estrogen metabolism to promote 2-methoxtestradiol-induced apoptosis in hen ovarian cancer. J Nutr Biochem. 2017;42:117-25. Epub 20170123. doi: 10.1016/j.jnutbio.2017.01.002. PubMed PMID: 28178600; PubMed Central PMCID: PMC5360509.
- Longcope C, Gorbach S, Goldin B, Woods M, Dwyer J, Morrill A, et al. The effect of a low fat diet on estrogen metabolism. J Clin Endocrinol Metab. 1987;64(6):1246-50. doi: 10.1210/jcem-64-6-1246. PubMed PMID: 3571427.
- Lord RS, Bongiovanni B, Bralley JA. Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev. 2002;7(2):112-29. PubMed PMID: 11991791.
- Coughlin SS, Stewart J, Williams LB. A review of adherence to the Mediterranean diet and breast cancer risk according to estrogen- and progesterone-receptor status and HER2 oncogene expression. Ann Epidemiol Public Health. 2018;1. Epub 20180316. doi: 10.33582/2639-4391/1002. PubMed PMID: 31008451; PubMed Central PMCID: PMC6474371.
- Li J, Tang X, Xu J, Liu R, Jiang L, Xu L, et al. HMGCR gene polymorphism is associated with residual cholesterol risk in premature triple-vessel disease patients treated with moderate-intensity statins. BMC Cardiovasc Disord. 2023;23(1):317. Epub 20230624. doi: 10.1186/s12872-023-03285-w. PubMed PMID: 37355634; PubMed Central PMCID: PMC10290797.
- Karimi E, Tondkar P, Sotoudeh G, Qorbani M, Rafiee M, Koohdani F. A personalised diet study: The interaction between ApoA2 -265T > C polymorphism and dietary inflammatory index on oxidative and inflammatory markers and lipid profile in patients with type 2 diabetes mellitus: A cross-sectional study. Int J Clin Pract. 2021;75(7):e14178. Epub 20210501. doi: 10.1111/ijcp.14178. PubMed PMID: 33759320.
- damska-Patruno E, Bauer W, Bielska D, Fiedorczuk J, Moroz M, Krasowska U, et al. An Association between Diet and MC4R Genetic Polymorphism, in Relation to Obesity and Metabolic Parameters-A Cross Sectional Population-Based Study. Int J Mol Sci. 2021;22(21). Epub 20211107. doi: 10.3390/ijms222112044. PubMed PMID: 34769477; PubMed Central PMCID: PMC8584592.
- Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuniga O, Delgado-Lista J, Gutierrez-Mariscal FM, Perez-Martinez P, et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol A Biol Sci Med Sci. 2012;67(1):3-10. Epub 20111020. doi: 10.1093/gerona/glr167. PubMed PMID: 22016358
- Strickland FM, Hewagama A, Wu A, Sawalha AH, Delaney C, Hoeltzel MF, et al. Diet influences expression of autoimmune-associated genes and disease severity by epigenetic mechanisms in a transgenic mouse model of lupus. Arthritis Rheum. 2013;65(7):1872-81. doi: 10.1002/art.37967. PubMed PMID: 23576011; PubMed Central PMCID: PMC3735138.
Share:
Related Posts

Goodbye Pie Chart, Hello Phase 1 Sliders
Written by Allison Smith, ND | 2025 As we usher in a new era of DUTCH testing which leaves behind the concept of the three-way

Introducing the DUTCH Dozen
Written by Kelly Ruef, ND | 2025 Hormone testing can be complex, which is why Precision Analytical developed the DUTCH Dozen, an interpretive framework that

DUTCH Report Enhancements
Written by Hilary Miller, ND | 2025 Precision Analytical have released the newest version of the DUTCH Test. This is the report’s most significant update

Gallbladder Health 101: What It Does and How to Keep It Working Well
Written by Ashley Palmer & Pooja Mahtani | 2025 The gallbladder may not get much attention compared to the gut, but it plays a central

Can You Bring Vitamins on a Plane? How To Travel with Supplements
Written by Austin Ruff | 2024 Are you traveling for a work conference, an athletic competition, or a weekend getaway? Just because you’re leaving home
